Combustion and its Types- Chemistry Guide for Class 8
Information about Combustion and its Types
Title | Combustion and its Types |
Class | Class 8 |
Subject | Class 8 Chemistry |
Topics Covered |
|
We already know that materials which produce heat energy on burning in air are called fuels. Materials, like coal, coke, kerosene, LPG, petrol and wood, are all fuels as they all produce heat on burning. This heat energy is used for various purposes. Some materials produce a flame on burning; some others do not. A candle, for example, produces a flame on burning. There are other materials which burn without a flame. Let us study the chemical process of burning and the types of flame produced during this process.
Combustion
You have learnt that when magnesium ribbon is heated in the presence of air, it catches fire and produces heat and light.
Similarly, when a piece of paper is burnt, it produces heat and light. We know that coal too burns in air producing heat and light.
- The materials, which on heating in the presence of air or oxygen, catch fire easily and produce heat and light energy, are called combustible materials.
- The chemical process in which a substance burns in air, or oxygen, with the release of heat and light energy is called combustion.
- The substance that undergoes combustion is called a combustible substance or fuel.
- A fuel may be solid, liquid or gaseous.
Conditions required for Combustion
Let us perform some activities to know about the different conditions required for combustion.
Activity 1
- Fix a candle on a table.
- Light the candle and observe the flame.
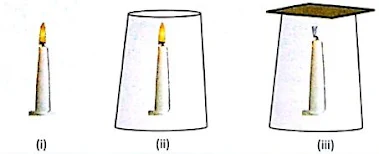
- Now, take a glass chimney and place it as shown in the figure.
- Observe the candle flame carefully.
- Now, place a cardboard on the top of the chimney.
- Observe the flame.
In the first case (Fig. i), the candle burns smoothly, because of sufficient supply of air from the atmosphere.
In the second case (Fig. ii), the candle flame flickers and becomes sooty, as there is insufficient supply of air.
In the third case (Fig. iii), the candle flame is put off because the supply of air is completely cut off.
From the above observations, we conclude that air is necessary for combustion. Oxygen, present in air, helps in the combustion of a fuel. It is, therefore, called a supporter of combustion.
Let us perform another activity to learn about the other conditions required for combustion.
Activity 2
- Take two paper cups.
- Half fill one of the cups with water and keep the other empty.
- Take two spirit lamps and put these two paper cups, (one empty and the other half-filled) above the flame carefully.
What do you observe?
You will observe that the empty cup catches fire immediately. However, the paper cup containing water does not catch fire immediately and can be held for quite some time on the flame. This is due to the fact that the temperature of empty paper cup rises fast, till it reaches the stage where it catches fire.
In case of the other paper cup, half filled with water, the temperature does not rise that fast as the water present also absorbs some heat. Hence, this cup does not catch fire.
- A piece of paper burns quickly when a burning matchstick is brought near it. On the other hand, a piece of wood cannot be burnt with the help of a matchstick.
- These observations tell us that different substances catch fire at different temperatures.
- The lowest temperature (minimum temperature), at which a (combustible) substance catches fire, is called its ignition temperature, or kindling temperature.
Let us look at some examples to realise that a substance must be heated up to its 'ignition temperature' for it to undergo combustion.
- We can boil water in a paper cup on a spirit lamp. It is because a part of the heat energy, supplied to the paper cup, passes on to water. As a result, the ignition temperature of paper is not achieved and it does not catch fire.
- Ignition temperature of white phosphorus is 35°C. During summer, room temperature often rises to above 35°C. White phosphorus then catches fire spontaneously.
- A matchstick can be lighted by striking its tip on a rough surface. The tip of the matchstick is made up of red phosphorus along with some other chemicals. On striking, friction generates enough heat to light the matchstick by making the chemical catch fire. The wood, used in the matchstick, is also of a particular kind; its ignition temperature is attained by the heat produced at the tip of the lighted matchstick.
- The flame of a burning candle goes off when we blow over it strongly. It is because the
(i) air current lowers the temperature of burning wax vapours below their ignition point.
(ii) the carbon dioxide, in our breath, acts as a fire extinguisher. Thus, the flame goes off.
We can now conclude that for producing, or sustaining, combustion the following three conditions are necessary:
- There must be a combustible substance.
- There must be a continuous supply of oxygen or air.
- The temperature should be above the ignition temperature of the combustible material.
Types of Combustion
Different types of combustion are:
- Slow Combustion
- Rapid Combustion
- Spontaneous Combustion
- Explosive Combustion
1. Slow Combustion
When a combustible material burns at a slow, or moderate rate, its combustion is called slow combustion.
Slow combustion usually occurs when there is an insufficient supply of air. Therefore, such a combustion never gets really completed. Burning of cowdung cakes, wood, etc. are examples of slow combustion.
2. Rapid Combustion
When a combustible substance burns at a fast rate, its combustion is called rapid combustion. Gaseous fuels generally undergo rapid combustion.
Rapid combustion occurs when there is a sufficient supply of air. The combustion, in such cases, is complete.
3. Spontaneous Combustion
When a combustible substance catches fire on its own, even at room temperature, its combusion is referred to as spontaneous combustion.
A piece of white phosphorus, or a piece of sodium metal, are observed to undergo 'spontaneous combustion' when kept in air.
4. Explosive Combustion
When a mixture of a combustible material and air burns completely, in a very short span of time in a closed space, an explosive combustion can take place. The combustible material, in such a case, burns uncontrollably in free supply of air and releases a very large amount of heat. This often produces the sound of an 'explosion'.
For example, when a cracker is ignited, a sudden and rapid reaction takes place, as a result, there is an evolution of heat, light and sound energy. A reaction accompanied by (an explosive) sound, is called explosion.
Important Points
- Substances which have a low ignition temperature and hence, catch fire easily with a flame are called inflammable substances.
- Food is a fuel for our body. Energy is produced during the 'combustion' of food.
- In pure oxygen, a substance burns five times faster than in air.
- More than 5,000 years ago, Egyptians made matchsticks by coating small pieces of pine wood with sulphur. These caught fire easily when they were rubbed strongly with each other. As a result of such rubbing, friction raised the temperature of sulphur above its ignition temperature and made it catch fire. This, in turn, ignited the pine wood pieces.
- Many of the forest fires are caused by human negligence. People living near the forest, may not extinguish the burning wood (etc.) after cooking, or sometimes, they may carelessly throw a burning matchstick on dry grass. Such acts of carelessness can lead to forest fires. )
- Sun produces an enormous amount of heat and light energy. 98 per cent of sun's mass is made of hydrogen at an extremely high temperature. At such temperatures hydrogen atoms can combine to form helium atoms and in the process release an enormous amount of energy in the form of heat and light. Energy produced by the sun in one hour is about 1020 times more than the total energy produced by burning 1000 kg of coal.