Solutions of Science CBSE Sample Paper 2018 Board Exam| Class 10th
Answer Sheet of CBSE Sample Papers for 2017-18 Final Exam. Solutions of Science Sample Paper is given here.
Section-A
2.
Pepsin
|
Trypsin
|
| 1. Produced in stomach 2. Acts in acidic medium |
1. Produced by pancreas 2.Acts in basic medium |
3. Atomic number of X = Mass number of X – No. of neutrons = 35-18 = 17
Electronic configuration = 2, 8, 7
Group number = 17, Period No. = 3
4.

5. i) Our demand for energy is increasing to improve quality of life and growth
of population
ii) Fossil fuels are limited.
6. Electric generator
Principle electormagenetic induction which states that electric current is
induced in a closed circuit because of changing magnetic field.

OR
i) Earth wire in electrical instruments saves us from all possible electric
shocks.
ii) Accidently, when live and neutral wires of an electric circuit comes into
direct contact, it is called shor circuiting.
iii) (a) 5A (b) 15A
7.

Maximum current through 4 Ω resister,

Maximum current through each 8 Ω resister = 1/2 × 2 = A
8. a) In the electrolysis of water, the gas collected at cathode is: Hydrogen
The gas collected at anode is: oxygen
b) The gas which is collected in double the amount during the electrolysis of water is Hydrogen. This is because water contains two parts of hydrogen element as compared to one part of oxygen
element by volume.
c) Pure water is a bad conductor of electricity, by adding drops of sulphuric acid; we make it a good conductor of electricity.
9.
Mendeleev’s Periodic table
|
Modern periodic table
|
| 1. The elements were arranged according to increased atomic Masses. | 1. The elements were arranged according to increased Atomic numbers. |
| 2. Position of isotopes was not Justified. | 2. There was no problem in the Placing of isotopes. |
| 3. Position of hydrogen was not Justified because it resembles Both with Alkali metals and Halogens. | 4. Hydrogen has been given a unique position due to its resemblance with alkalis and Halogens. |
10. There are two ways of anaerobic breakdown of glucose. First step is breakdown of glucose molecule into pyruvate which takes place in cytoplasm.
The anaerobic breakdown in bacteria is called fermentation. During fermentation pyruvate is broken down to ethyl alcohol and carbondioxide.
When there is lack of oxygen in our muscle cells pyruvate is broken down to lactic acid. Very less amount of energy is released in both the above cases.
OR
Arteries carry blood away from the heart while veins carry blood towards the
heart.Arteries are thick walled while veins are thin walled.
Valves are absent in arteries while valves are present in veins to ensure that blood flows in one direction only.
11. Mendel conducted a monohybrid cross with pea plants, and he observed that one of the contrasting characters disappears in F1 generator. This character reappears in F2 generation (obtained by selfing F1) in just 25% of the progeny.
Mendel conclude that the character which epresses itself in F1 is the dominant character while the other one when is not able to epress thourhg present in F1 individuals is recessive. This recessive character is able to express only in its pure form i.e. in 25% of F2 individuals.
12. (i) u = 50 - 26 = 24 cm
v = 74 - 50 = 24 cm
∴ 2f = 24 cm
∴ f = 24/2 = 12 cm
ii) u = 50 - 38 = 12 cm
i.e. Candle is at f
∴ Image is formed at infinity.
iii)

13 Any three point:
• Plants and animals are pH sensitive. Living organisms can survive only in narrow range of pH change.
• pH of the soil. Plants require a specific pH range for their healthy growth.
• pH in our digestive system. Our stomach produces hydrochloric acid that helps in the digestion of food. During in digestion the stomach produces too much acid that cause pain and irritation.
• Change in pH causes tooth decay. Tooth decay start when the pH of the mouth is lower than 5.5. Tooth enamel gets corroded when the pH in the mouth is below 5.5.
• Self-defense by plants and animals through chemical warfare. Bee–sting leaves and acid causing pain and irritation. Applying a mild base like baking soda on the stung area provides relief.
OR
The name of the compound is Plaster of Paris.Its chemical formula is CaSO4. ½ H2O.
Equation:
CaSO4.2H2O ⟶ CaSO4. ½ H2O + 1½ H2O
It is used in the hospitals mainly as plaster for supporting fractured bones in the right position
14. Fossils provide evidence in favour of evolution/establish evolutionary relationships by providing missing links.
Two ways:
(i) Relative method – Fossils found closer to the surface are more recent than those in deeper layer.
(ii) By detecting the ratios of different isotopes of the same element in the fossils material.
15 a) Viral/STD
HIV
b) Senstivity and awareness among the citizens towards leading a healthy and fit life.
16. Activity (Refer circuit diagram given below)
Take a battery (12 V), a variable resistance (or a rheostat), an ammeter (0–5A), a plug key, and a long straight thick copper wire. Insert the thick wire through the centre, normal to the plane of a rectangular cardboard. Take care that the cardboard is fixed and does not slide up or down. Connect the copper wire vertically between the points X and Y, as shown in diagram in series with the battery, a plug and key. Sprinkle some iron filings uniformly on the cardboard. Keep the variable of the rheostat at a fixed position and note the current through the ammeter. Close the key so that a current flows through the wire. Ensure that the copper wire placed between the points X and Y remains vertically straight. Gently tap the cardboard a few times. Observe the pattern of the iron filings. It is observed that the iron filings align themselves showing a pattern of concentric circles around the copper wire.
These represent the magnetic field lines.

i) Right hand thumb rule
ii) Yes, Alpha particles being, positively charged constitutes a current in
the direction of motion.
No, Neutron being electrically neutral constitute no current.
17.

OR
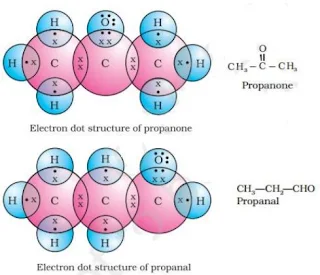
18. a) (i)

ii) Correct labelling
iii) Medulla controls blood pressure, salivation and vomiting.
Cerebellum controls precision of voluntary movements and equilibrium.
b) Over production of growth hormone leads to gigantism and it’s underproduction leads to dwarfism
19 a) Myopia
b) f = -1/45 = -0.22 cm
Concave lens
c)

d) Causes
i) Due to excess curvature of eye lens
ii) Elongation of the eye ball.
20. a) The arrangement of metals in the vertical column in the order of decreasing reactivity is called reactivity series or activity series.
A metal placed above hydrogen in the activity series will displace hydrogen from water or acids. A metal placed at the top of the activity series would displace metal below it. Thus a more reactive
metal displaces a less reactive metal from its salt solution.
b)
i) For obtaining metals that are in the middle of the reactivity series, oxides of such metals can be reduced with coke (carbon) which acts as a reducing agent.
Example: 2 Fe2O3 + 3 C ⟶ 4 Fe + 3CO2
ii) For obtaining metals that are high in the reactivity series, their oxides are reduced to metals by the process of electrolysis
Example: electrolysis of sodium chloride at cathode: Na+ + e- ⟶ Na
At anode: 2Cl- ⟶ Cl2 + 2e-
21. a)
• More use of dispossible items like paper plates, plastic items, polythene etc.
• Changes in packaging.
Suggestion – Reuse of polythene bages, plastic containers.
b) Hewk
Biomaginification
OR
a) Scientific soil and water conservation is called watershed management. Advantages:
i) Increases production and income of watershed community.
ii) Mitigates droughts and floods.
iii) Increases the life of downstream dams reservoirs.
b)
i) Maximum level of bio magnification occurs here because of progressive accumulation.
ii) We get very small amount of energy as only 10 % of the previous energy gets transferred at each trophic level
Section B
22. i) Acetic acid will remain colourless in phenolphthalein
ii) Acetic acid will dissolve in distilled water forming a clear solution
iii) Universal indicator gives orange colour with acetic acid.
iv) Sodium hydrogen carbonate will give brisk effervescence due to the formation of CO2 gas.
23. Set I will have more length of foam because it consist of soft water.
Set II will form less foam because it consist of hard water due to the presence of CaSO4.
24. Budding in Yeast

25. i) The set up should be airtight.
ii) Germinating seeds (living) should be used
26. Graph
V = 9V - 5V = 4V
I = 2.65A - 1.40A = 1.25 A
R = V/I = 4./1.25 = 3.2Ω
OR
An ammeter has 10 divisions between 0 to 0.5A.
So,
1 Division = 0.5A/10 = 20
17 divisions = 17/20 = 0.85A
27.

