Competency Based Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations
According to new CBSE pattern, competency based questions as wells as Case Based Questions will be asked in the board examination. Students need to get familiar with these new types of questions in order to get good marks. We are providing CBQ questions for class 10 science chapter 1 chemical reactions and equations.
Chapter 1 Chemical Reactions and Equations CBQ Questions
Question 1. Which of the following is an example of simple displacement?
- the electrolysis of water
- the burning of methane
- the reaction of a metal with an acid
- the reaction of two salt solutions to form a precipitate
Answer
3. the reaction of a metal with an acid
Question 2. Which of the following is a NECESSARY condition for ALL chemical reactions?
- The reactants should be in the same state.
- Energy should be supplied to the reactants.
- The reactants should be at the same temperature.
- There should be physical contact between the reactants.
Answer
4. There should be physical contact between the reactants.
Question 3. Given below is the balanced chemical equation for the thermal decomposition of lead nitrate.
2Pb(NO3)2 → 2PbO + 4NO2 + O2
Which of the following information does the coefficients of PbO and NO2 in the equation (2 and 4 respectively) tell us?
- the ratio of the number of moles produced of the two substances
- the ratio of the number of atoms in the two substances
- the ratio of the mass produced of the two substances
- the ratio of the densities of the two substances
Answer
4. the ratio of the number of moles produced of the two substances
Question 4. The diagram below shows the set-up in which electrolysis of water takes place.
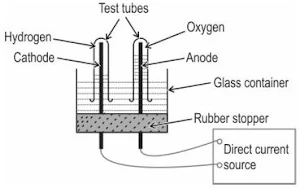
(a) What type of reaction takes place?
(b) Explain why this is an example of an endothermic reaction?
(c) The test tube containing hydrogen is removed carefully from the apparatus. A lit match stick is brought near the mouth of this test tube. The gas burns with an explosive "pop" sound.
Write a balanced chemical equation for this reaction and indicate whether energy is absorbed or released.
Answer
(a) Decomposition / Electrolytic decomposition
(b) Energy in the form of electrical energy is absorbed during the decomposition of water.
(c) Balanced equation:
2H2O + energy → 2H2 + O2
Question 5. Eight identical, iron blocks are placed on the ground in the two arrangements X and Y as shown below. The block arrangements are kept moist by sprinkling water every few hours.
Which of the arrangements is likely to gather more rust after ten days? Justify your answer.
Answer
- arrangement Y
- Rusting is a surface phenomenon.
- Arrangement Y has a larger surface area exposed to air.
Question 6. The following chemical equation does not represent a chemical reaction that can take place.
3 Fe (s) + 4 H2O (l) → Fe3O4 (s)
State what needs to be changed in the equation above for it to represent the correct reaction between Fe and H2O.
Answer
The water should be in the form of steam, not liquid.
Question 7. Trupti mixes an aqueous solution of sodium sulphate (Na2SO4) and an aqueous solution of copper chloride (CuCI2).
Will this lead to a double displacement reaction? Justify your answer.
Answer
There will be no reaction.
- All the ions will be in solution.
- There is no insoluble product formed on mixing the two solutions.
Question 8. Dilip was comparing combination reactions with decomposition reactions.
Which class of chemical substances may be the product of a decomposition reaction but NOT a product of a combination reaction?
Answer
element
Question 9. Write the balanced chemical equation of any one reaction that CANNOT be classified as combination, decomposition, simple displacement or double displacement.
Answer
CH4 + 2O2 → CO2 + 2 H2O
6 CO2 + 6 H2O → C6H12O6 + 6 O2
Question 10. Tina finds a paper covered with a white substance in a chemistry lab. She keeps the paper near the window of the lab and comes back to pick it up after five hours to take it home. She noticed that the white substance had turned grey.
(a) What could be the most likely substance on the paper that Tina found?
(b) The substance changed from white to grey. Write the chemical equation for this reaction.
(c) State ONE application of this property of the substance seen in daily life.
Answer
(a) silver chloride (AgCI)/silver bromide (AgBr)
(b) 2AgCl → 2Ag + Cl2
OR
2AgBr → 2Ag + Br2
(c) in black and white photography

